



The group of Theoretical and Computational Chemistry is part of the Physical Chemistry division, and is supported by the Department of Chemistry of the University of Crete (UC), and the Institute of Electronic Structure and Laser (IESL), Foundation for Research and Technology, Hellas (FORTH).
 Quantum Chemistry (I, II),
Quantum Chemistry (I, II), Mathematics for chemists, and
Mathematics for chemists, and  Computers and Computational Chemistry.
Computers and Computational Chemistry.
 Dynamics and spectroscopy of vibrationally highly excited small
polyatomic molecules.
Dynamics and spectroscopy of vibrationally highly excited small
polyatomic molecules.
 Construction of analytical Molecular Potential Energy Surfaces from ab initio
calculations and empirical data.
Construction of analytical Molecular Potential Energy Surfaces from ab initio
calculations and empirical data.
 Studies of the structure, dynamics, and thermodynamical properties of
atomic and molecular microclusters.
Studies of the structure, dynamics, and thermodynamical properties of
atomic and molecular microclusters.
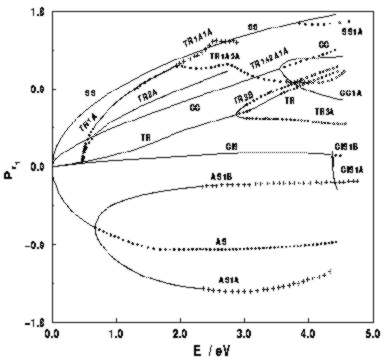 [1]
and phosphaethyne (HCP)
[1]
and phosphaethyne (HCP)  [2]
are prototypes for studying Quantum Chaos and the
correspondence of classical to quantum mechanics.
[2]
are prototypes for studying Quantum Chaos and the
correspondence of classical to quantum mechanics.
The main research effort of the Theoretical and Computational Chemistry Group in Crete is focused in understanding the dynamics of vibrationally excited small polyatomic molecules and in the study of the structures, dynamics and thermodynamics of small and intermediate size atomic and molecular clusters. The driving force for the undertaken projects is our interest to investigate the non-linear mechanical properties of molecular species and the way they manifest themselves in spectroscopy and chemical reactivity.
The molecular Potential Energy Surfaces (PES) in the Born-Oppenheimer approximation are strongly non-linear functions, and thus, the study of dynamics in the classical mechanical approximation requires the application of the theory of Non-Linear Mechanics. This inevitably brings the question of the correspondence between classical and quantum mechanics and related problems of Quantum Chaos. In spite of the fact that there is still no satisfactory theory of Quantum Chaos the good correspondence of classical mechanical stationary objects (Periodic Orbits, Tori, Stable and Unstable Manifolds, Cantori) to the quantum mechanical eigenfunctions found in numerous calculations allows us to apply the systematic techniques of Non-Linear Mechanics to Molecular Dynamics and to extract precious information about the spectroscopy and dynamics of relatively large molecular systems.
Our group has contributed into this field through
the development of the Periodic Orbit Method (POM), which is a
powerful tool for extracting
the dynamics from vibrational spectra and predicting sometimes the
existence of exotic states in the molecules which are difficult to foresee
with the old spectroscopic models.
A review article with recent results of this project is given in
 [3].
[3].
Another field of our research activity is the study of
small atomic and molecular clusters. Water clusters were the first systems
which attracted our interest not only because of the importance of water
in our planet, but also, this species allows us to investigate the dynamics
of hydrogen bonding
systems. It is well known that clusters bridge the gap between
isolated molecules and the macroscopic states. However, clusters may also
show unique properties not encountered in large scale bodies.
It was a surprise for us to find that small water clusters form stable
cubic structures which of course have not been seen in ice 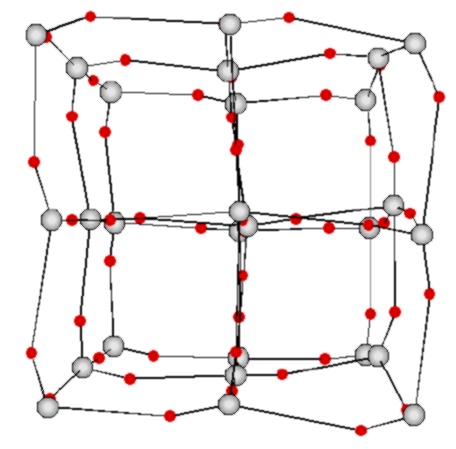 [4].
[4].
Inert gas aggregates doped with metal cations are prototypes for
investigating solvation effects. Molecular beam experiments and laser
photodissociation spectroscopy for these systems are carried out with a
collaborating experimental group of IESL-FORTH. Molecular dynamics
calculations not only reveal the structures of the most stable clusters
(magic numbers) which are detected in the mass spectra, but they
may also reveal the existence of interesting
isomeric forms such as solvated and non-solvated Mg+ in Argon
 [5]and
Paper[6].
[5]and
Paper[6].
For this project it is necessary to construct PESs. This is usually
achieved by carrying out accurate ab initio calculations for the small
clusters and then employing a model for the larger ones. Thus, this
field of Chemical Dynamics brings in collaboration quantum chemists, molecular
dynamicists, and experimentalists
 [7].
[7].
 H
H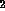 System.
System.
[2] H. Ishikawa, R. W. Field, S. C. Farantos, M. Joyeux, J. Koput,
C. Beck and R. Schinke.
HCP - CPH Isomerization: Caught in the Act.
Annual Review of Physical Chemistry, 50:443, 1999.
[3] S. C. Farantos.
Exploring Molecular Vibrations with Periodic Orbits.
Int. Rev. Phys. Chem., 15:345, 1996.
[4] A. Vegiri, and S. C. Farantos.
Classical Dynamics of Hydrogen Bonded Systems: Water Clusters.
J. Chem. Phys., 98:4059, 1993.
[5] G. Fanourgakis, and S. C. Farantos.
Potential Functions and Static and Dynamic Properties of
 and
and
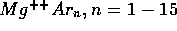 clusters.
clusters.
J. Phys. Chem., 100:3900, 1996.
[6] G. Fanourgakis, and S. C. Farantos, P. Parneix, and Ph. Brechignac.
Isomerization Processes in Mg^+Ar_{12} cluster:
A comparison of anharmonic and harmonic models}
J. Chem. Phys., 106:4954, 1997.
[7] G. C. Fanourgakis, S. C. Farantos, Ch. Luder, M. Velegrakis and S. S. Xantheas.
Photofragmentation Spectra and Structures of Sr+Arn, n=2-8 Clusters: Experiment and Theory
J. Chem. Phys., 109:108, 1998.
Tel : 0030-81-39 1813 (personal )
Tel : 0030-81-39 1301 to 1303 (secretary)
Tel : 0030-81-23 8468 (Chemistry)
Fax : 0030-81-39 1305
URL : http://TCCC.iesl.forth.gr/
Elm : farantos@iesl.forth.gr
*******************************************************
SCF