Daniel W. Zaharevitz, Frederick Biomedical Supercomputing Center, Developmental Therapeutics Program, PRI/DynCorp
A very important part of drug design is predicition of small molecule binding to a target macromolecule. A detailed quantitative predicition of small molecule binding can require sophisticated computational techniques and lots of computer time. Often a reasonable qualitative prediction of binding can be made by just specifying the spatial arrangement of a small number of atoms or functional groups. Such an arrangement is called a pharmacophore [1]. A pharmacophore can be inferred from an analysis of active molecules ( "active analog approach" [2-4] ) or can be built from knowledge of the structure of the target macromolecule. A common use of pharmacophores is to search 3D databases for molecules that contain the pharmacophore. In the best cases not only does this result in the discovery of new active molecules without the need for testing large numbers of compounds, but it also can give a structurally diverse set of active molecules [5-7].
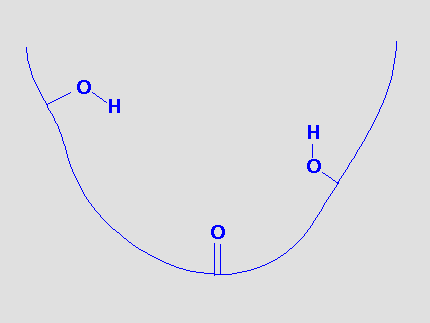
Suppose a binding site can be represented by the following model:

There are obvious sites for hydrogen bonds and we could postulate that a molecule that could bind to this site would provide functional groups that could hydrogen bond to the receptor functional groups.
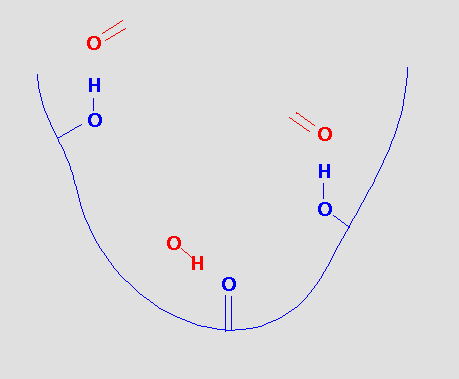
which gives us the pharmacophore
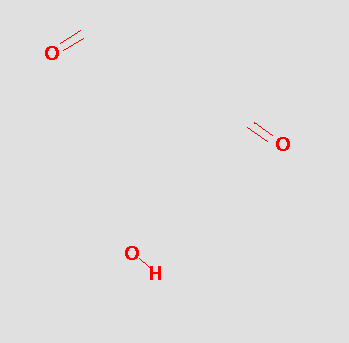
The pharmacophore might be tested by searching a database and hits like the following might be obtained:
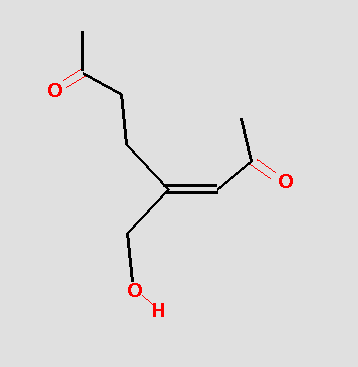
The pharmacophore search finds molecules with different overall chemistries, but which have the functional groups in the correct geometry. From a drug development point of view this is very useful because the overall chemistry of the molecule may play an important role in formulation, bioavailability, toxicity, etc. The more chemical classes of molecules represented in the actives, the greater chance that a useful clinical agent can be found.
Of course the preceding illustration is vastly oversimplified. In reality, targets and active molecules are three-dimensional objects and therefore so are pharmacophores. For a detailed example of building and searching a 3D database see the page on the NCI DIS 3D database.